Specialists in Imaging
for Clinical Research
Spire Sciences brings unparalleled experience and expertise in central image analysis to your clinical trials.
Helping bring new therapies into clinical use faster
Our team of specialized radiologists and imaging scientists is dedicated to imaging for clinical trials, and has helped bring numerous new therapies to regulatory approval. Our imaging experts and specialized reading systems ensure that your study achieves the highest reading accuracy and efficiency possible, out-performing other central imaging services.
We value close working relationships with our sponsors' development teams and bring decades of experience and insight to ensuring the success of clinical trials that use medical imaging.
We provide the most accurate image analyses, trustworthy scientific advice and beneficial innovations to reduce uncertainty, time and cost in therapeutic development.
Pharma's Radiology Department
Unlike other providers that offer imaging as only one of many services, we specialize in imaging. Radiology for clinical trials is all we do.
About Spire
Our mission is to improve world health by helping bring new and better therapies into clinical use faster through excellence in imaging for clinical trials.
In contrast to contract research organizations and core labs that offer image analysis as only one of many services, Spire Sciences is focused exclusively on central image analysis. Our expert radiologists are among the most experienced and accomplished in the world, and our reading processes and systems are the most sophisticated and error free in the industry, providing unsurpassed reading performance across multiple therapeutic areas.

Charles Peterfy, MD, PhD
Founder and Chief Executive Officer
Charles Peterfy, MD, PhD
Founder and Chief Executive Officer
Dr. Peterfy is an internationally recognized leader in imaging for clinical trials, and has been developing, validating and implementing innovative imaging techniques for clinical trials for over 35 years. Over that time, Dr. Peterfy founded multiple organizations providing imaging services for clinical trials and advancing research and education in this unique application of radiology, and developed numerous imaging outcome measures, imaging techniques and positioning devices that have become standards in clinical trials in key diseases, including inflammatory arthritis, degenerative joint disease and certain tumors.
Leader in Clinical-Trial Imaging
Over 35 years pioneering imaging techniques and endpoints for clinical trials
Founded multiple organizations advancing imaging for clinical trials
Personally read thousands of images for clinical trials across a variety of diseases
Academic and Professional Background
American Board certified radiologist, with fellowship training from UCSF
PhD in Pharmacology and Therapeutics from McGill University
Radiology faculty at UCSF
Former Director of Arthritis and MRI Research for OARG at UCSF
Former Chair of Imaging Working Group for NIH Osteoarthritis Initiative
Former member of Board of Directors, Intersocietal Accreditation Commission
Co-Chair of MRI Working Group, OMERACT (Outcome Measures in Rheumatology)
Key Contributions
Co-founded Synarc Inc., the largest imaging core lab globally, currently Clario
Founded Spire Sciences Inc., focused on excellence in imaging for clinical trials in specific disease areas
Co-founded ISEMIR (International Society for Imaging in Rheumatology)
Developed multiple imaging techniques and endpoints that have become standards for clinical trials in inflammatory arthritis, osteoarthritis and certain tumors
Education
MD, McGill University, Canada (1985)
PhD, Pharmacology & Therapeutics, McGill University (1989)
American Board of Radiology certification (1991)
Radiology fellowship, University of California San Francisco (UCSF) (1992)
Professional Activities
Director of Arthritis and MRI Research for Osteoporosis and Arthritis Research Group at UCSF (1992 - 2000)
Co-founder, Chief Scientific Officer, Executive VP of Synarc Inc. (1998 - 2009)
Largest imaging core lab in the world
Became Clario in 2021
Co-founder, President of ISEMIR (International Society for Imaging in Rheumatology) (2006 - 2021)
Non-profit organization advancing research and education
Founder, CEO of Spire Sciences Inc. (2009 - present)
Focusing on excellence in imaging for clinical trials of specific disease
Board of Directors, IAC (Intersocietal Accreditation Commission) (2016 - 2021)
Non-profit organization establishing standards and guidelines for accrediting MRI facilities throughout the US
Innovations and inventions for clinical trials imaging
Inflammatory arthritis
Syn-X-RA and newer X-Frame
For reproducible, side-verified X-ray of hands, wrists and feet
The standard in X-ray trials of rheumatoid arthritis for past 25 years
RAMRIS (Rheumatoid Arthritis MRI Scoring)
In conjunction with the OMERACT (Outcome Measures in Rheumatology Clinical Trials) MRI Working Group
The standard in MRI trials of rheumatoid arthritis for past 22 years
Syn-M-RA and newer M-Frame
For reproducible, side-verified MRI of hands and wrists
The standard in MRI trials of rheumatoid arthritis for past 21 years
CARLOS (Cartilage Loss Score)
The standard in MRI trials of rheumatoid arthritis for past 10 years
PsAMRIS (Psoriatic Arthritis MRI Scoring)
In conjunction with the OMERACT MRI Working Group
The standard for MRI trials of psoriatic arthritis for past 10 years
Osteoarthritis
MRI quantification of articular cartilage in knee and hand
First validated method for quantifying cartilage with MRI (1994)
Fixed-flexion X-ray technique
The standard for X-ray trials in osteoarthritis trials for past 25 years
SynaFlexer and newer FixedFlexionFrame and reference standard
For reproducible fixed-flexion X-ray, with magnification correction
The standard for X-ray trials in osteoarthritis trials for past 25 years
WORMS (Whole Organ MRI Scoring)
Most widely used multi-feature MRI assessment for MRI trials of osteoarthritis for past 20 years
Fixed-location joint-space narrowing measurement for knee osteoarthritis
Outperforms conventional X-ray measurements
HOAMRIS (Hand Osteoarthritis MRI Scoring)
In conjunction with the OMERACT MRI Working Group
The standard for MRI trials of hand osteoarthritis for past 10 years
Oncology
TVS (Tumor Volume Score) for Tenosynovial Giant Cell Tumor (TGCT)
The standard for TGCT trials for past 10 years
Modified RECIST (Response Evaluation Criteria for Solid Tumors) for TGCT
The standard for TGCT trials for past 10 years
TDS (Tissue Damage Scoring)
The standard for TGCT trials for past 10 years
Automated tumor response indicating measurement tool
Reducing variability and error in RECIST assessments
Automated follow-up tumor selection verification
Reducing variability and error in tumor measurement
Publications and committee work
Authored 2 medical textbooks and over 200 scientific articles and book chapters
Chaired Imaging Working Group for NIH Osteoarthritis Initiative
Public-private partnership, and largest natural history study of osteoarthritis ever conducted
Co-chair MRI Working Group, OMERACT (Outcome Measures in Rheumatology)
International organization developing standards for trials using MRI
American College of Rheumatology (ACR) Rheumatoid Arthritis Clinical Trials Task Force
Chair, Standardizing Radiography and CT, Imaging as a Biomarker: Standards for Change Measurements in Therapy
National Institute of Standards and Technologies (NIST)
Harmonization of Imaging Review Charters and Integration of Imaging in Therapeutic Development
Drug Information Association (DIA), Food and Drug Administration (FDA)
Participated in numerous other standardization committees and advisory boards
Charles Peterfy, MD, PhD is an American Board certified radiologist with fellowship training from University of California San Francisco (UCSF) and a PhD in Pharmacology and Therapeutics from McGill University. He was attending radiologist at UCSF for seven years where he also served as Director of Arthritis and MRI Research for the Osteoporosis and Arthritis Research Group, which conducted numerous clinical trials and epidemiological studies through the university.
In 1998, Dr. Peterfy co-founded Synarc Inc, which became the largest imaging core lab in the world by 2000. He served as its Chief Scientific Officer and Executive Vice President for 11 years. As Synarc grew, it expanded beyond imaging to include a broad range of services for clinical research, and in 2021 it became Clario, a general CRO.
In 2009, Dr. Peterfy left Synarc, and formed Spire Sciences, to bring the focus back to imaging, particularly excellence in imaging for diseases that needed special expertise, and where he felt Spire could make a significant positive impact and out-perform other imaging core labs.
Spire Sciences succeeded in those objectives, as evidenced by having been trusted to read the majority of clinical trials worldwide that used imaging in those key therapeutic areas for the past 15 years, having developed many of the outcome measures and imaging techniques that have become the standards for clinical trials in those areas.
Dr. Peterfy was the first to develop and validate a technique for quantifying articular cartilage in the knee and hand using MRI 30 years ago. He developed the fixed-flexion X-ray technique and positioning frame (SynaFlexer and the newer Fixed Flexion Frame), which has been the most widely used method for radiographically evaluating the knee in osteoarthritis for more than 25 years. He also developed fixed-location joint-space width measurement for monitoring osteoarthritis progression more sensitively than possible with conventional minimum joint-space width measurement. He also developed the Whole Organ MRI Scoring method, WORMS, which has been the most widely used multi-feature MRI assessment method for evaluating osteoarthritis in epidemiological studies and clinical trials for over 25 years.
In 2002, Dr. Peterfy in conjunction with the OMERACT (Outcome Measures in Rheumatology Clinical Trials) MRI Working Group, which he co-chairs, developed the RAMRIS (Rheumatoid Arthritis MRI Scoring) method for evaluating rheumatoid arthritis in clinical trials. He also co-developed PsAMRIS (Psoriatic Arthritis MRI Scoring) and HOAMRIS (Hand Osteoarthritis MRI Scoring). These methods have been the most widely used for evaluating inflammatory arthritis, and are currently the standards for clinical trials using MRI. Dr. Peterfy also developed CARLOS (Cartilage Loss Score), the most commonly used method for monitoring cartilage loss in rheumatoid arthritis with MRI, and he invented the Syn-X-RA and X-frame positioning devices for error-free radiography of the hand and foot in arthritis, along with the Syn-M-RA and M-frame devices for reproducible MRI of the hand and wrist in arthritis trials.
He chaired the Imaging Working Group of the NIH Osteoarthritis Initiative, one of the first public-private partnerships between NIH and the pharmaceutical industry and the largest natural history study of osteoarthritis ever conducted, and he supervised the collection and central quality control of the images for that historic study, which are available to the public worldwide for clinical research.
In 2006, he co-founded the International Society for Imaging in Rheumatology, ISEMIR, a non-profit organization aimed at advancing imaging in rheumatology through research and education, and he served as its President until it was shut down in 2021 in the wake of the Covid pandemic.
He also served on the Board of Directors of the Intersocietal Accreditation Commission (IAC) from 2016 to 2021, establishing standards and guidelines for accrediting imaging facilities throughout the US.
In Oncology, Dr. Peterfy developed several outcome measures specially designed for Tenosynovial Giant Cell Tumor (TGCT), arguably the most difficult tumor of all to measure longitudinally in clinical trials because of its diffuse nature, highly irregular shape, ill-defined margins, local invasiveness and asymmetrical growth. These measures include modified-RECIST for TGCT, the Tumor Volume Score (TVS) and the multi-feature Tissue Damage Scoring method (TDS), which out-performed RECIST 1.1 and expand on it in clinically meaningful ways. Using these methods, Spire Sciences has read the images for most if not all trials of TGCT globally over the past 11 years, for which effective therapies, sometimes even curative, are now available in the clinic, when before the options available to these patients were extremely limited.
Dr. Peterfy has authored two textbooks and over 200 scientific articles and book chapters, and participated in numerous standardization committees, scientific advisory boards and expert panels for advancing imaging in clinical trials.

Caroline Reinhold, MD, MSc
Vice President, Oncology
Caroline Reinhold, MD, MSc
Vice President, Oncology
Dr. Reinhold is an internationally recognized leader in oncological and gynecological imaging, and has been developing, validating, and implementing oncological imaging techniques, and classification and reporting imaging systems used world-wide for over 25 years.
Leader in clinical trials imaging
Over 25 years pioneering imaging techniques, biomarkers and classification systems for clinical trials
Expert in artificial intelligence, with numerous peer-reviewed publications and lectures on the accuracy, ethics, governance, and implementation of AI in medical imaging
Extensive experience leading large, multicenter imaging clinical trials
Personally read thousands of images for clinical trials for oncological and gynecological indications
Professional background and key contributions
American Board certified radiologist, with fellowship training from Yale University
MSc in Epidemiology and Biostatistics from McGill University
Professor and former Interim Chair, Department of Radiology at McGill University
Associate Chair of Research, Department of Radiology at McGill University
Awarded over $8M in grant funding from prestigious agencies
Co-founder and Director of the Augmented Imaging Precision Health Laboratory (AIPHL) at the Research Institute of the McGill University Health Center
Co-Chair of the Ovarian-Adnexal Reporting & Data System (O-RADS) of the American College of Radiology
Former Vice Chair, MRI track of the Ovarian-Adnexal Reporting & Data System (O-RADS) of the American College of Radiology
MRI lead for the International Federation of Gynecology and Obstetrics (FIGO) Menstrual Disorders Adenomyosis subcommittee
Former Director of the Clinical Trials Imaging Unit at the Research Institute of the McGill University Health Center
Former Medical Director of Oncology, Synarc Inc., the largest imaging core lab in the world (became Clario in 2021)
Education
MD, McGill University, Canada (1984)
American Board of Radiology Certification (1990)
Abdominal Imaging Fellowship, Yale University (1991)
MSc, Epidemiology and Biostatistics, McGill University (2000)
Innovations in clinical trials imaging
Co-developed a standardized reporting/classification system for MRI-based diagnosis of uterine adenomyosis
With the International Federation of Gynecology and Obstetrics (FIGO) Menstrual Disorders Adenomyosis subcommittee
Co-developed a standardized lexicon and risk stratification system for the MRI classification of adnexal masses (O-RADS MRI)
With the American College of Radiology
Co-developed automated RECIST response indicating measurement tool
Spire Sciences
Co-developed automated follow-up tumor selection verification
Spire Sciences
Leadership roles
Medical Director of Oncology, Synarc Inc. (1999 - 2010)
Largest imaging core lab in the world
Became Clario in 2021
Associate Chair of Research, Department of Radiology at McGill University (2015 - present)
Secured over $8M in grant funding from prestigious agencies
Vice Chair, MRI track of the Ovarian-Adnexal Reporting & Data System (O-RADS) (2016 - 2023)
American College of Radiology
MRI lead for the International Federation of Gynecology and Obstetrics (FIGO) Menstrual Disorders Adenomyosis subcommittee (2018 - present)
Interim Chair of the Department of Radiology at McGill University (2019 - 2021)
Director of the Clinical Trials Imaging Unit at the Research Institute of the McGill University Health Center (2019 - 2021)
Co-founder and Director of the Augmented Imaging Precision Health Laboratory (AIPHL) (2019 - present)
Research Institute of the McGill University Health Center
Co-chair of the Ovarian-Adnexal Reporting & Data System (O-RADS) (2023 - 2025)
American College of Radiology
Vice President of Oncology, Spire Sciences Inc. (2014 - present)
Publications and committee work
Authored 4 textbooks and over 350 scientific articles and book chapters
Participated in numerous standardization committees, scientific advisory boards and expert panels advancing imaging for clinical trials in oncology and gynecology
Caroline Reinhold, MD, MSc is Vice-President of Oncology at Spire Sciences. Dr. Reinhold is an internationally recognized leader in oncological and gynecological imaging, and has been developing, validating, and implementing oncological imaging techniques, and classification and reporting imaging systems used world-wide for over 25 years. She has extensive experience in leading large multicenter imaging clinical trials both for industry and academia. She is also an international recognized leader in the field of artificial intelligence with numerous peer-reviewed publications and has lectured on the accuracy, ethics, governance, and implementation of AI solutions in medical imaging.
Dr. Reinhold is an American board-certified radiologist with fellowship training from Yale University and an MSc in Epidemiology and Biostatistics from McGill University. She is Associate Chair of Research for the Department of Radiology at McGill University, co-founder and director of the Augmented Imaging Precision Health Laboratory (AIPHL) at the Research Institute of the McGill University Health Center, prior interim Chair and Director of the Clinical Trials Imaging Unit at the Center of Innovative Medicine at the Research Institute of the McGill University Health Center. She is currently also co-chair of the Ovarian-Adnexal Reporting & Data System (O-RADS) of the American College of Radiology, and is the MRI lead for the International Federation of Gynecology and Obstetrics (FIGO) Menstrual Disorders Adenomyosis subcommittee.
In 1999, Dr. Reinhold joined Synarc Inc. (currently Clario Inc.), which was at the time the largest imaging core lab in the world, and she served as its Medical Director of Oncology for 10 years. In that capacity she developed Synarc's oncology service line. In 2010, Dr. Reinhold returned to academia and clinical radiology, but consulted and read clinical trials images for Spire Sciences. In 2024, she became VP of Oncology for Spire Sciences to extend its superior reading processes and systems to a broader range of oncological indications.
Dr. Reinhold has authored 4 textbooks and over 350 scientific articles and book chapters, and participated in numerous standardization committees, scientific advisory boards and expert panels advancing imaging for clinical trials in oncology and gynecology.

Peter Countryman, PhD
Chief Operating Officer
Peter Countryman, PhD
Chief Operating Officer
Dr. Countryman is Chief Operating Officer at Spire Sciences, and has worked with Dr. Peterfy and Dr. Reinhold for 3 decades on developing clincial trial imaging processes and systems.
Systems Expert in Clinical Trial Imaging
29 years experience in Clinical Trial Imaging development and operations
Expert systems design for central image quality control and analysis
Expert in international regulations and security for imaging in clinical trials
Academic and Professional Background
PhD in Physics from Stanford University (1988)
Faculty at UCSF, developing image analysis software for clinical trials at OARG
Original member at founding of Synarc (now Clairo)
Senior Software Architect, then Product Manager
Original member at founding of Spire Sciences
Scientific Director, now Chief Operating Officer
Key Contributions and Developments
Developed Synarc's medical image database/archive system (IMDA)
Led development of systems for NIH Osteoarthritis Initiative
Developed systems infrastructure for Spire Sciences, including PACS, relational databases, and image reading/reporting software
Developed improved image quality and reconstruction methods for PET
Created a virtual environment with cloud-hosted servers and VPN access for Spire Sciences
Peter Countryman, PhD is Chief Operating Officer at Spire Sciences. Dr. Countryman received his PhD in Physics from Stanford University and worked for several years in the field of PET imaging, making use of his knowledge of nuclear medicine instrumentation and his scientific programming skills to improve the image quality of a sodium-iodide based PET system, and to develop an image reconstruction technique for PET with limited projections.
In 1995, he joined the faculty of the University of California San Francisco (UCSF) and oversaw the development of viewing software for clinical trials conducted by the Osteoporosis and Arthritis Research Group (OARG).
He joined Synarc (now Clario) at its founding in 1998 as Senior Software Architect, and quickly advanced to Product Manager, serving as liaison between the scientific staff and the engineering department. He played a major part in the design and development of Synarc's medical image database/archive system (IMDA), and led the development of the systems supporting the NIH Osteoarthritis Initiative (OAI). In 2009, Dr. Countryman joined Spire Sciences at its founding, and served as Senior Scientist and Scientific Director before becoming Chief Operating Officer. He developed the computing infrastructure at Spire, including Spire's PACS (Picture Archival and Communications System), relational databases and software for reading images and producing reports. Spire Sciences functions in a virtual environment, with all servers hosted in the cloud, and all users accessing the data through a virtual private network.
Dr. Countryman is also expert in international regulations and security with respect to Clinical Trial Imaging.
Explore our Services
Our focus is accuracy in image analysis, as this is the most important determinant of statistical power in clinical trials. We accomplish this by integrating unparalleled excellence in image reading with meticulous quality control and experienced multi-site protocol design.
Integrating key elements of imaging
Image acquisition
Image analysis
Protocol Design
Spire Sciences' experts have designed hundreds of imaging protocols for clinical trials and have the scientific and technological knowledge and the practical hands-on experience needed to turn the scientific needs of a study into practical reality.
Training & Quality Control
Image analyses can be only as accurate as the quality of the images is able to support. Spire Sciences has trained hundreds of sites around the world and knows what it takes to produce the highest-quality images for central analysis.
Disease Areas
Oncology
Solid Tumors
Solid Tumors with Immune-based Therapy
Tenosynovial Giant Cell Tumor
Hematology/Lymphoma
Reporting and Data System (RADS)
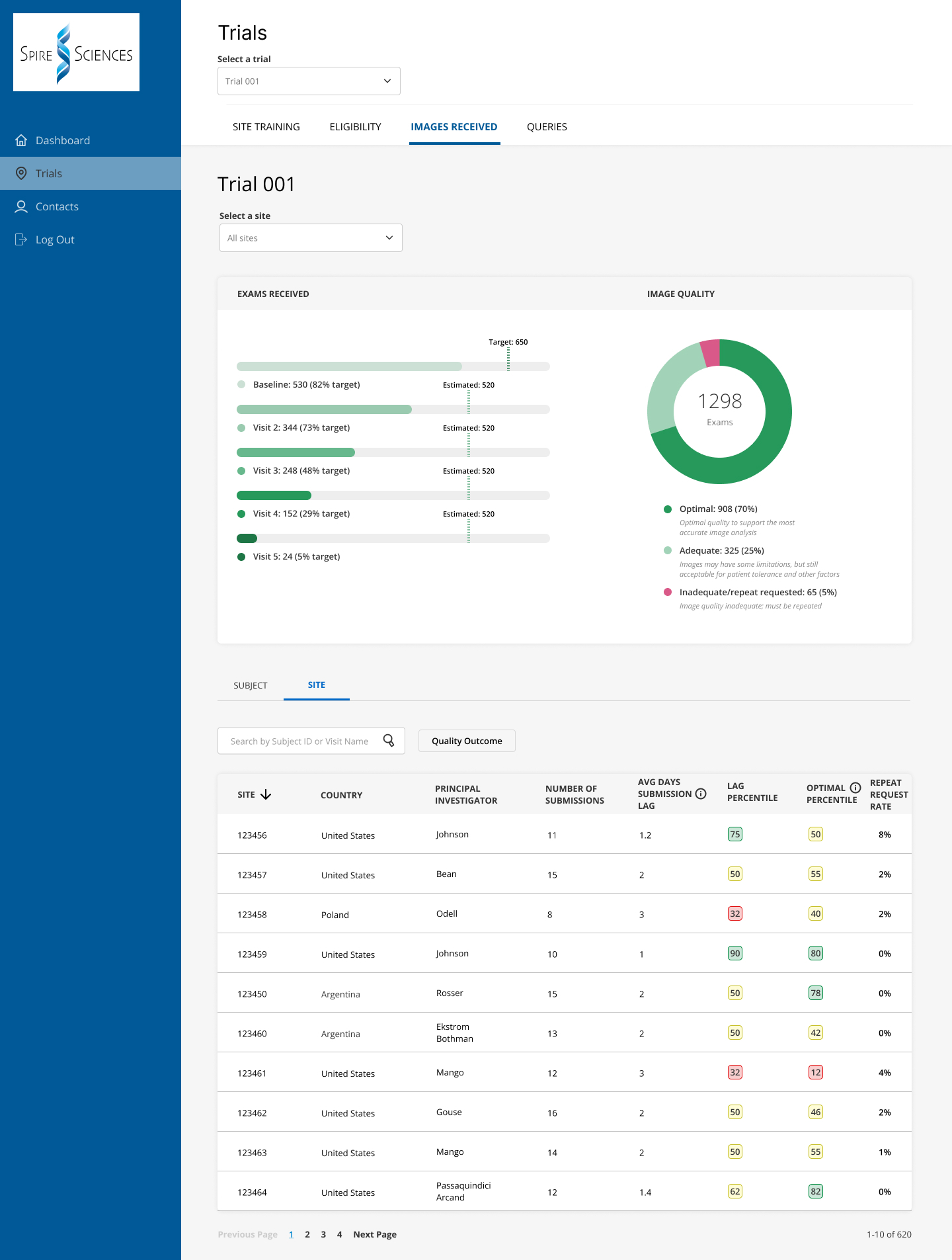
Spire Portal
Spire's sponsors, their CROs and participating study sites receive access to Spire's online Portal, where they can find up-to-date study progress and site performance data. Individual sites can see their own performance results and compare those to aggregate data from all the sites, so they can see where they rank with respect to patient enrollment, screening failure rate, image quality metrics, query resolution, and other relevant parameters.
Managing site performance has become increasingly important, as worldwide shortages in imaging technologists, radiologists and other staff make it more challenging for clinical facilities to support research.
Charles Peterfy
